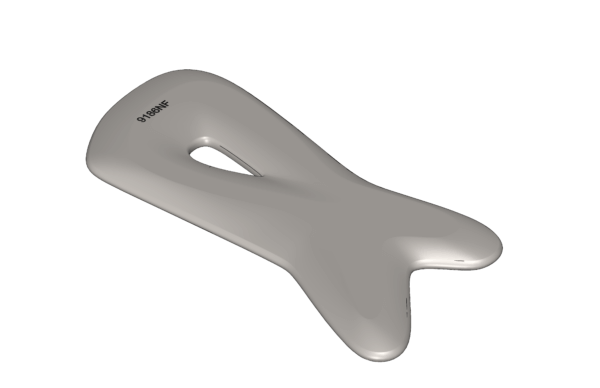
Pause Facetool
A patented tool made from medical-grade stainless steel to help stimulate fibroblasts, collagen-producing cells within the fascia connective tissue. It’s shaped to follow your contours and, when gently stroked over skin, it may help improve blood flow, support cell turnover, remove waste, boost collagen production and optimize overall skin nutrition, which can help minimize sagging and enhance volume and elasticity.
Pause Fascia Stimulating Tool

The Client
Rochelle had worked with another design firm that developed an initial surface model of her fascia stimulation tool in Rhino. She needed help with design for manufacturing, material selection, and process selection, as well assistance getting to market as a medical device.
The Challenge
The fascia stimulating tool project started with some challenges. This included selecting a durable, skin-safe material that met functional and aesthetic requirements. Identifying a manufacturing partner capable of producing the device efficiently and cost-effectively added complexity. Navigating the FDA regulatory pathway further complicated development, particularly as Rochelle aimed to claim specific therapeutic indications. Balancing compliance with the regulatory framework while maintaining affordability and high-quality production proved critical in overcoming these multifaceted hurdles.
The Solution
- Reviewed the design to understand the core design intent and indications for use.
- Determined an appropriate material (304 stainless steel).
- Determined an efficient manufacturing process for the part (investment casting) and tapped our manufacturing network to support a build of 10,000 initial units.
- Performed a regulatory assessment and determined class 1 510(k)-exempt regulatory pathway for the device to support the client’s desired indications of use and marketing claims.
- Built a quality management system tailored to the device and business.
- Supported the process of bringing the investment casting manufacturer into FDA compliance with documentation, labeling procedure, and registration support.
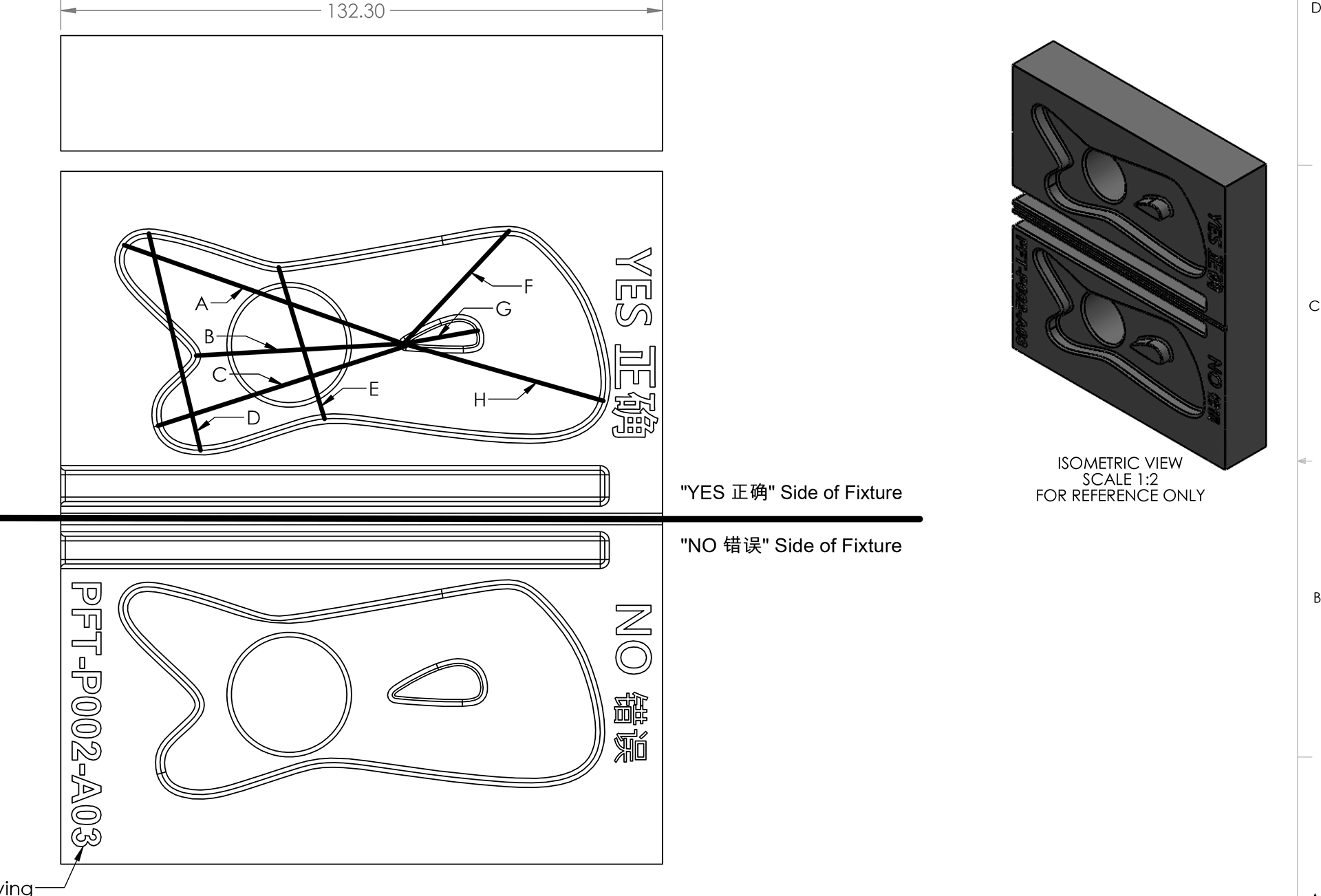
- Implemented minor DFM tweaks to support the investment casting process and implemented laser-etched lot numbers on all devices to provide traceability.
- Managed the manufacturing startup, negotiation, sampling, inspection, and acceptance processes.
- Interfaced with client’s vendors to receive custom packaging materials coming from other locations to receive, and perform the work of final product packaging (box components, leather case, user manual, stainless tool, and shrink wrap) and inspection.
The Results
Successfully manufactured and delivered the initial 10,000 fascia stimulating tools to Rochelle’s New Jersey fulfillment center. Established a robust quality management system and secured FDA registration, ensuring compliance and product quality. These milestones marked a significant step toward scaling the product and meeting market demands effectively.