Contents (click to jump to each section):
- What is human factors engineering?
- What is a User Interface (UI)?
- Basic Concepts of Human Factors Engineering
- Example: USB design change makes a big difference
- Importance of Human Factors Engineering for Medical Devices
- Featured Kickr Design Project: LIT Medical Device
What is Human Factors Engineering (also known as ergonomics and usability engineering)?
Within the scope of product development, human factors engineering (HFE) is an approach to design that focuses on and optimizes all the interactions between the user and devices. It can be applied to hardware, software, and complex systems in many ways. HFE accounts for the strengths and weaknesses of humans during the design of interactive products to ensure efficiency and safety by reducing the risk of user error. Ergonomics and usability engineering (UE) are some other common terms that can be used interchangeably with human factors engineering.
Human factors engineering helps match your product to customer expectations
Whether you’re developing a high-tech device or simple consumer product, you need to consider the human factors before investing in a large manufacturing order. Neglecting human factors in your design could cause problems down the road, the most common being device misuse which can lead to all sorts of unintended consequences. Even if the product is working exactly as it is designed, customers may decide that it doesn’t work at all because they can’t figure out how to use it or it becomes unsafe due to misuse. You can minimize these issues by working with a product development team that is knowledgeable on the subject matter of human factors engineering and usability engineering.
What is a User Interface (UI)?
Human factors engineering puts a large focus on User Interfaces (UI). User Interface describes the means and methods that a user interacts with a device. A well-designed UI makes a device’s controls easy to understand and use. To understand the elements of a UI, it’s useful to think about the ways that users will:
- Interpret information from the device
- Understand the information and make choices on what to do
- Manipulate the device and adjust its components and/or its controls (e.g. changing configuration, replacing a part, or turning the device on/off)
In addition to the user’s input to the device it’s also important to think about the way the device will:
- Receive inputs from the user
- Respond and provide feedback about the effects of their inputs
Basic Concepts of Human Factors Engineering
Human factors engineering is a very deep and wide subject, so I broke down some of the high-level concepts that are usually considered in the process to help you better understand the general process:
User Research – The process begins by researching and understanding who exactly will be using your product and what their unique characteristics are. These characteristics include physical coordination/dexterity, sensory abilities (sight, hearing, etc.), cognitive abilities (i.e. memory), literacy, mental and emotional state, and willingness to learn to name a few.
Environmental Considerations – Beyond understanding the user, it is also imperative to understand the user’s surroundings when they are interacting with the device. Is the product going to be stored in a small space? Is it going to be exposed to harsh elements? Does it need to be chemically resistant to perform? Does the device need to interface with other devices, hardware or software? Again, these set of considerations are often completely unique to each product, so there is no silver bullet for discovering these key attributes.
Human Anatomy/Physiological Considerations – As humans, we have a shared set of unique characteristics. It is paramount to design your products with the human anatomy in mind. Is your device comfortable to hold correctly? Is it too heavy for the users to lift? Can the UI be easily seen during use? Is the shape of the device easy to grasp?
Cognitive & Mental Considerations – The purpose of designing a great UI is to reduce the mental effort needed by users to use the device. When we use something that is not intuitive in its design, we begin to feel stress, noticeable or not. Depending on the application, this cognitive load can make or break the effectiveness of a device. Is your device difficult to understand? Does it require reading a long list of instructions before using? Does interacting with it feel like second nature or does it feel like jumping through hoops? After all, devices are tools, and tools are meant to aid us in our tasks not add complication and unnecessary frustration.

Example: USB-A to USB-C, a small design change that makes a big difference in usability
Think of the last few times you tried plugging in a standard USB cable (technically referred to as USB-A) into a computer or outlet. Did you get it right on the first try? Why are they so hard to plug in? If you are like most humans, you have experienced the frustration of flipping the cable back and forth multiple times just to get it configured correctly and plugged in. Perhaps you decided to take the time to look closely at each USB connector before plugging in, using your eyes and brain to decipher the puzzle. Even then, you may find that you still got it wrong, leaving you thinking, “who in the world designed these things?”
Don’t get me wrong, the Universal Serial Bus (USB) is one of the greatest inventions of the 90’s (in my opinion) and the overall usefulness of the technology far outweighs the minor setback mentioned above. However, to bring my point back to human factors and ergonomics, the brilliant minds who continue to innovate USB technology took note and obviously applied HFE principles to their latest design with the introduction of USB-C in 2014.
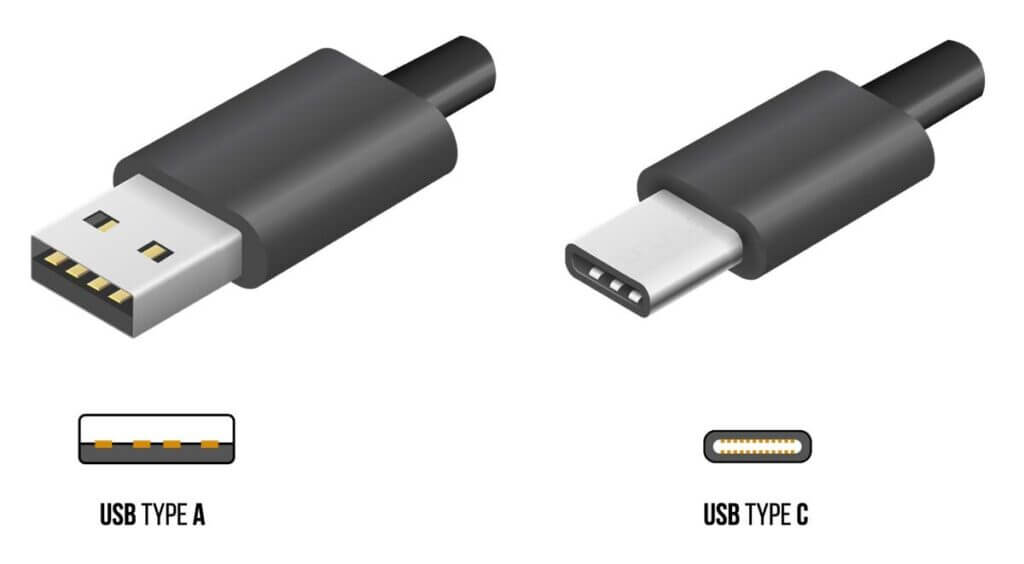
The main difference I want to highlight between the standard USB-A plug and the USB-C is the reversible design. Rather than having just one orientation for connecting, the USB-C can be connected in two different orientations, making it much easier to plug it in and move on with your life. Users no longer must spend their precious mental effort plugging in their devices, and they will have more left in the tank to focus on their work. The designers effectively eliminated a method of misuse. To some, this may seem like a minute and insignificant improvement, but considering how many USB devices are used around the world, the amount of time and frustration saved by the reversible design is incalculable and priceless.
Apple® was the first major player to adopt a reversible connector, and the same concept applies to the evolution between their original iPod® sync cable and the Lightning® connector they released in 2012. To put the ubiquitous influence of USB in perspective, all of Apple®’s iPod® sync cables and Lightning® cables include either a USB-A or USB-C connection on the other end.
The Importance of Human Factors Engineering for Medical Devices
Now that you have a broad overview of some principles of HFE, let’s take a deeper look into HFE’s role in in healthcare and medical devices. In these applications, the most important goal of the HFE process is to minimize use-related hazards and risks through design. Unlike most consumer products, making mistakes when using a medical device can result in serious implications for not just the users, but their patients. The FDA understands the importance of HFE and usability for medical devices, and they have laid out multiple guidance documents to explain the usefulness of the process and how to approach it. Here are some examples of “use-related hazards” outlined by the FDA:
- Device use requires physical, perceptual, or cognitive abilities that exceed the abilities of the user;
- Device use is inconsistent with the user’s expectations or intuition about device operation;
- The use environment affects operation of the device and this effect is not recognized or understood by the user;
- The particular use environment impairs the user’s physical, perceptual, or cognitive capabilities when using the device;
- Devices are used in ways that the manufacturer could have anticipated but did not consider; or
- Devices are used in ways that were anticipated but inappropriate (e.g., inappropriate user habits) and for which risk elimination or reduction could have been applied but was not.
Depending on the medical device type, HFE documentation and validation may be required for new medical device approval. If you’re developing a new medical device, the earlier you begin accommodating for these considerations, the better off you’ll be.
For more in-depth information directly from the FDA in regards to medical devices, here are some helpful links:
Do you need to develop a product with human factors engineering principles? Our team of designers and engineers are prepared to walk you through the process. Contact us for a Free Consultation & Estimate for your next project.
Apple®, iPod®, and Lightning® are registered trademarks for Apple, Inc